21st Century Doctors
Reprogramming Cancer: Turning Tumors into Healthy Brain Cells
by Ireri del Moral | October, 2024
Around 7000 people live with medulloblastoma, most of them children under the age of 10. This very malignant brain cancer offers limited chances of survival. Sadly, those who do survive often face lifelong disabilities due to aggressive treatment. Luckily, there seems to be another option in the works! According to recent research, hope lies in thyroid hormone.
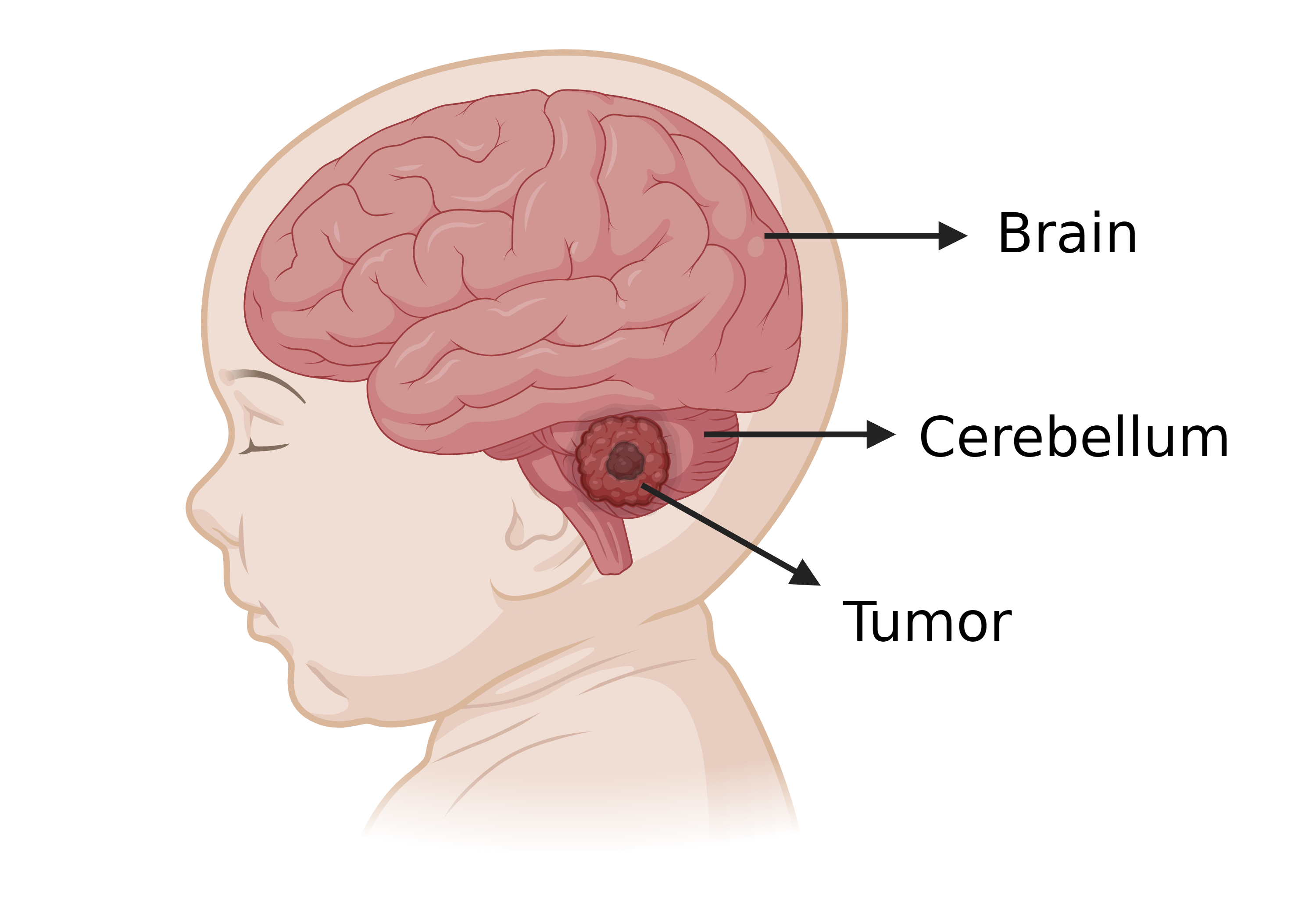
Each year, approximately 1000 people worldwide, predominantly children, are diagnosed with medulloblastoma. This brain cancer forms a malignant tumor in the cerebellum, the region in charge of coordinating balance and movement. Therefore, symptoms can be very severe.
Medulloblastoma is a, mainly pediatric, brain cancer that forms a tumor in the cerebellum, the region that coordinates balance and movement. Created in BioRender.com
Conventional treatment, which includes surgery, radiotherapy, and chemotherapy, has remained unchanged for over 40 years. Unfortunately, it is ineffective for some and particularly aggressive for growing children. Only around 60% of patients survive, and they often face long lasting disabilities. Since cancer is basically a group of damaged cells that multiply out of control, they need to be stopped. Because of this, current methods aim to destroy malignant cells, but in doing so, they also damage and kill healthy cells, directly reducing survivors’ quality of life – the “lesser” of two evils.
With the stakes so high for young patients, the Yang Lab at the Fox Chase Cancer Center (FCCC) in Philadelphia, is focused on developing better strategies to treat brain tumors, including medulloblastoma. Dr. Anahí Valdés, a postdoctoral researcher in this group, says that there hasn’t been a breakthrough in 25 years. To make matters worse, this cancer has four different subtypes, each reacting differently to treatment. As a result, the candidate treatments do not work adequately for all, and those that do may be too toxic.
However, the lab’s recent findings published in “Cancer Cell” in august 2024, suggest a potential breakthrough involving thyroid hormone. Dr. Yijun “Boris” Yang, leader of this research, observed that brain cells that grew in an environment where thyroid hormone was present, matured faster. Intrigued, he injected T3, the active form of this hormone, into a lab mouse with medulloblastoma. When he returned the following Monday, he found that the mouse, unlike the others, had eaten normally. This led him to believe that T3 might have had some beneficial effect on the tumor. Additionally, previous research also shows that this hormone is crucial for cerebellum development. All this could not be just a coincidence, so Boris and his group set to work.

The Yang Research Lab at FCCC, Philadelphia. From left to right: MSc. Allie Heller, former member; Dr. Anahí Valdés, Postdoctoral Researcher; Dr. Yijun “Boris” Yang, Assistant Research Professor; and Zeng-jie Yang, Principal Researcher. Source: https://yangresearchlab.org/photos/
First, they treated aggressive medulloblastoma cells with T3. Surprisingly, even the smallest dose reduced the growth of cancer cells. This didn’t cause cell death but rather promoted their conversion into neurons, a type of brain cells. “Instead of killing tumor cells, we normalize them,” said Dr. Zeng-jie Yang, Principal investigator of the lab to FCCC News. This process is known as differentiation therapy. This method aims to “retrain” cancer cells rather than destroying them and has shown great promise in cancer treatment.
Building on these positive findings, Dr. Boris Yang and his team took the next step. They implanted medulloblastoma cells in the brains of laboratory mice and treated them with T3. These cells lost their ability to form tumors. Nonetheless, researchers were concerned that T3 could cause heart problems. But even when exceeding the recommended dose “by a thousand times”, according to Dr. Valdés, no adverse effects are observed. In fact, T3 treatment not only shrank tumor sizes but also improved overall health and survival without causing weight loss in mice. This suggests it could be a safe and effective treatment. After all, “…our goal is to better their quality of life, not just to make them survive longer,” Dr. Valdés rightly says.
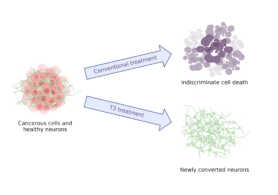
Differentiation therapy using T3 turns cancerous cells into brain cells. This aims to limit the indiscriminate destruction of healthy and malignant cells. Created with BioRender.com
We must keep in mind however, that while these are promising results, the treatment is still experimental. Both researchers acknowledge that there are still many questions to answer. This includes evaluating the effect of T3 in combination with chemo, verifying that these newly converted brain cells work, or whether this treatment acts differently between boys and girls.
Fortunately, we may soon find out, since work on these projects is already underway. In fact, Dr Boris’ exciting, and soon to be published findings show, among other things, that chemo doses can be reduced in combination with T3. This reduces the aggressiveness and toxicity of treatment while also preventing tumor regrowth.
They are also preparing to initiate clinical trials in collaboration with Seattle Children’s Hospital and the Pediatric Neuro-Oncology Consortium by early 2025. “This is our dream,” shared Dr. Boris Yang.
This progress is the result of years of dedicated work. Dr. Yang says that they try balancing hard work with enjoyment and always celebrate each other’s achievements at the lab. And while not every day is thrilling, Dr. Valdés states, each day contributes to their ongoing research. Both researchers agree that it’s very exciting to see the whole story come together. At the end of the day, despite the challenges, their dedication and enthusiasm offer new hope for medulloblastoma patients and their families. The future of these treatments could radically change the way we deal with this challenging disease.
*This article was developed with the assistance of ChatGPT to help improve clarity and coherence. The final text was reviewed and edited by the author to ensure accuracy and appropriateness.
Sources:
Leading medulloblastoma to a differentiation end
Study Finds Thyroid Drug ‘Normalizes’ Tumor Cells in Pediatric Brain Cancer
Global trends, gaps, and future agenda in medulloblastoma research: a bibliometric analysis
You must be logged in to post a comment.