Oceans and CO2
Seashells inspire a CO2 storage solution
Each human adds on average 4 kg of CO2 in the seawater per day. This gas impacts oceans by changing their chemistry. It endangers animal species such as the mollusks that build the beautiful shells you are used to seeing during holidays. In this perspective, we need solutions to remove CO2 from the oceans. This year, a team of engineers from the University of California found a new technique, inspired by seashells, to stock CO2 in solid form. Is this new idea useful and viable? This single-step carbon-capture system is not yet used on a large scale but it brings promising features. Let’s explore that further, with the opinion of experts on ocean issues.

Last January, an American research team published a paper where they presented the technique they found by turning to the ocean. The process (Figure 1) begins with pumping water directly from the oceans into a rotating pierced tube where chemical reactions happen thanks to electricity. In the seawater, CO2 molecules are combined with water molecules and together they form carbonate. In this technique, carbonates are conduced to precipitate: be combined with calcium to result in solid particles called calcium carbonate (Figure 2). According to Olivier Sulpis, researcher at Utrecht University (Department of Earth Sciences), “the idea is to change the dissolved CO2 to something solid, thus allowing to store it more easily because the carbon is locked in small mineral particles”.
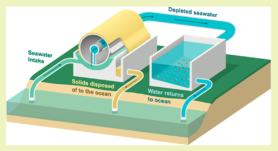
Figure 1. Illustration of the CO2 storage concept from the UCLA team.
Mollusks and many other organisms use a similar process to fix CO2 in order to build their own shells. They pump calcium and carbonate from seawater, concentrate them in their body where they are able to induce calcium carbonate formation. Afterwards, they assemble particles to form layers and make their shell thicker. As they are living organisms, their food gives them energy to induce these kinds of chemical reactions. Researchers aimed to reproduce these natural processes to find sustainable solutions. According to Mathilde Hagens, assistant professor at Wageningen University (Department of Environmental Sciences), “making use of your knowledge of natural processes for engineering purposes is a good thing to do. And this technique does that, so it’s what I like about it.”
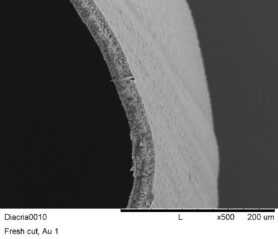
Microscopic view of the mollusk shell wall made of calcium carbonate (species: Diacria costata)
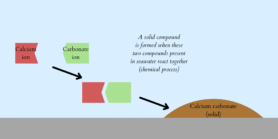
Figure 2. Explanatory diagram of carbonate precipitation.
This solution is interesting because it tries to be part of a sustainable system more than other techniques. For Dr. Mathilde Hagens, a promising side of this technique is that “it doesn’t need any additional chemicals and any additional energy because if you need a lot of energy to take up CO2 from the atmosphere then the net effect is smaller.” Indeed, an efficient solution to store CO2 should not at the same time produce a lot of CO2 during its use. First, as water contains 150 times more CO2 than air in the same volume, less energy is needed to remove it directly from oceans. Then, one of the products formed with the chemical reactions (di-hydrogen) could be used afterwards as a fuel to power electricity. Finally, engineers don’t want to add chemicals in the process. The idea is rather to break water molecules to obtain a new compound. This one will change seawater chemistry and conduce to accelerate chemical reaction to form carbonate. That’s why this carbon management solution may be capable of higher rates than existing Negative CO2 Emission Technologies (NETs) and it does not need more CO2 than what is stored.
However, there are still some points that need further research. A first concern is the possible effects on the coastal ecosystems when applying this kind of process. Pumping vast amounts of seawater with all the living-organisms is not without impact. It may be easy to remove all fishes and other visible animals from the seawater, but more steps will be necessary for the micro-organisms. A way to filter them at the entrance of the engine could be useful otherwise they might not survive. A second uncertainty is where the particles formed can be stocked. Releasing the particles back in the ocean might result in CO2 dissolving again. Therefore, it could be more sustainable to look for a way to reuse the solid products in the construction industry, as a kind of cement for example. Consequently, the fate of the elements produced will impact the cost of structures hosting this process and in that way, their implementation on a larger scale. This is why further studies are being conducted by the engineers team to be now tested outside the laboratory.

Still, this technique gives us a concrete solution to manage CO2. Indeed, this gas is harmful for us in the air as it contributes to global warming. It also damages the oceans, even if we cannot directly see the negative effects. By changing the chemistry of seawater, animals that make shells have more and more difficulties and need more energy to build them. “If there is more CO2 in the seawater, it will create an imbalance in the chemistry and there will be less carbonate ions available for them to produce their calcified structure.” (Dr. Paula Ramos-Silva, evolutionary biologist at Naturalis). This happens because dissolved CO2 is in equilibrium between 3 different shapes, including carbonate ions (Figure 3). If CO2 is in larger quantities, it will react with carbonate ions decreasing their presence in the seawater. That’s why it becomes urgent to reduce the amount of CO2 and store it in an environment where it won’t dissolve again, as this technique tends to do.
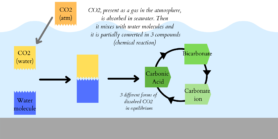
Figure 3. Explanatory diagram of CO2 dissolution in seawater.
To conclude, this technique could be a great innovation regarding the current issue of CO2 removal. Inspired by nature, it could be more efficient than other techniques (single-step concept) and carbon could be stored in a stable form. As we need to decrease the amount of CO2 in the oceans and in the atmosphere, any technique will be helpful because reducing our emissions will not be enough. “Today we emit so much CO2 that there is no solution that will solve the problem by itself. It will probably take a multitude of solutions, each of which will remove a little bit of the problem” (Dr. Olivier Sulpis). Let’s see in the next months how engineers will develop this technique to test it on larger scales and how it will help to improve the CO2 situation regarding Earth’s and oceans’ future!

If you want to know more about:
– this technique and the team that discover it
https://newsroom.ucla.edu/releases/using-seawater-to-reduce-co2-in-atmosphere
– the use of di-hydrogen as a fuel
https://www.energy.gov/eere/fuelcells/hydrogen-fuel-basics
– taking inspiration from nature (bio-mimicry)
https://biomimicry.org/hope-in-action-learning-from-nature-to-solve-big-problems/
– the ocean’s carbon balance
https://earthobservatory.nasa.gov/features/OceanCarbon
References:
– Saline Water-Based Mineralization Pathway for Gigatonne-Scale CO2 Management
Erika Callagon La Plante, Dante A. Simonetti, Jingbo Wang, Abdulaziz Al-Turki, Xin Chen, David Jassby, and Gaurav N. Sant
ACS Sustainable Chemistry & Engineering 2021 9 (3), 1073-1089
DOI: 10.1021/acssuschemeng.0c08561
– This carbon-capture tech removes CO2 from the ocean by making seashells
Adele Peters, Fast Company, World changing Ideas, 06/03/21
– Could the ocean hold the key to reducing carbon dioxide in the atmosphere ?
Wayne Lewis, Newsroom UCLA, 12/01/21
– Interviews with:
Dr. Mathilde Hagens, assistant professor at Wageningen University, Department of Environmental Sciences, Soil Chemistry and Chemical Soil Quality (PhD focused on ocean acidification, CO2 uptake due to climate change in the context of natural changes).
Dr. Paula Ramos-Silva, evolutionary biologist at Naturalis, Marie Skłodowska-Curie Postdoctoral Fellow, working on the project EPIC (Evolution of planktonic gastropod calcification).
Dr. Olivier Sulpis, postdoctoral researcher at Utrecht University, Marine Biochemistry, working in the geochemistry research group of the Department of Earth Sciences.
(Not able to get in contact with the UCLA engineering team to find more information about the research methods and the current status of this innovation.)
Photos credits:
– https://c1.staticflickr.com/9/8492/8297270187_84c2271195_b.jpg
– Diagram of the CO2 removal technique: UCLA Institute for Carbon Management https://pubs.acs.org/doi/full/10.1021/acssuschemeng.0c08561
– Microscopic view of the mollusk shell wall made of calcium carbonate: Dr. Paula Ramos-Silva
– https://pngimg.com/uploads/seashell/seashell_PNG4.png
– https://cdn.pixabay.com/photo/2017/03/01/17/17/beach-2109139_960_720.jpg
Notice to readers: further permission related to the material excerpted (Figure1. Illustration of the CO2 storage concept from the UCLA team) should be directed to the ACS.

