Immortality
Immortal Beasts and how they Work
Immortality, a lot of people think that this only exists in stories, that it is nonsense. But in reality it depends on what you understand by immortality. Rinny Kooi, retired doctor of biology and former lecturer at Leiden University, distinguishes two types of immortality: immortality through what you leave as a person, for example the 17th-century painter Rembrandt van Rijn. And immortality in the physical sense, as with some plants and animals. This article is about this last form of immortality.
There are several ways in which organisms in nature can be seen as immortal. According to Rinny Kooi, there are plant species that can make clones of themselves from new roots time and time again, such as the strawberry plant. There are fungi that make a whole network that continues to grow and continue to exist. Also, there are old spores in the ground that can develop centuries later.
There are also animals that can live for hundreds to thousands of years, such as the ocean quahog, a shellfish that can live up to 400 years, and the sponge.

Image 1: Deep-sea sponge forest (credit NOAA)
The oldest sponge found has reached an age of approximately 11,000 years. In addition, there are animals that can go back to their younger stages of life such as the “immortal jellyfish”. And recently scientists discovered that the Elysia sea slugs, such as Elysia atroviridis, can decapitate and regenerate themselves. Finally, there are animals, such as the tardigrade, that can survive extreme situations that we humans could never survive without protection.
But how do these forms of immortality work for the sponge, the immortal

Image 2: Monorhaphis chuni glass sponge skeleton
jellyfish, the Elysia and the tardigrade? And could we humans learn something from this, or maybe even use it in the medical world? Or to become immortal ourselves?
Image 3: Induced Pluripotent Stem Cells Show Potential for Autologous Stem Cell Therapies
The sponge, immortal jellyfish and Elysia are simple organisms that live in the sea. All three species appear to have pluripotent cells. This allows them to regenerate. More information about how pluripotent cells work can be found in our video. Although these cells work in the same way, the way of immortality is different in these three animals.
The sponge consists entirely of pluripotent cells, this remains the same throughout its life. This allows the sponge to not only regenerate, but also to replace old cells with younger ones throughout its life. This prevents mutations in the DNA and allows the sponge to live for thousands of years.

Image 4: immortal jellyfish
The immortal jellyfish can go back to its larval stage, the polyp, when it experiences stress from life-threatening situations such as starvation. This way he can survive those situations and later develop back into his medusa form (jellyfish form). In theory, this process can go on indefinitely. In reality, the immortal jellyfish is often eaten.
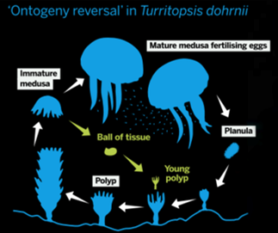
Immage 5: lifecycle immortal jellyfish

Image 6: decapicated Elysia (credits Sayaka Mitoh)
The regenerative capacity of the Elysia is similar to that of the sponge and the jellyfish. Special about this sea slug is that it has a slightly more complex body than the other two and can still survive with only its head.
For the tardigrade, it all works differently. Tardigrades are microscopic creatures that can be found everywhere and can survive many extreme situations, such as extreme high and low temperatures, radioactive radiation and even the vacuum of space. They are not built to live under these conditions, but they do have the ability to survive them. To do so, they have a rare survival strategy called cryptobiosis, or suspended animation. In this survival strategy, the metabolism is stopped and the tardigrade

Image 7: Een beerdiertje van de soort Paramacrobiotus kenlanus, zittend op een stukje mos. (credit European Atlas of Soil Biodiversity / Eye of Sciences)
loses almost all its water. Normally, this enormous waterloss would be deadly for an organism, but thanks to special sugars the tardigrade can survive. Because no more metabolism takes place, the tardigrade does not need water, oxygen or food. The disadvantage, however, is that he cannot do anything during the suspended animation. The tardigrade remains in this state of suspended animation, until the living conditions in its environment improve. They can maintain this for up to five years, sometimes even longer.
This is all very interesting, but how can we use it? Scientists have been working for years on techniques to convert specialized cells, such as skin cells, into “pluripotent” cells. It has also been possible to change a specialized cell directly into another type of specialized cell in mice by turning a number of genes “on”. These techniques could be used to make new tissues and cells, for example, to cure diseases, to grow organs for donation and to prevent aging. In 2020 it was even possible to convert cells from a blood sample into “pluripotent” cells in a 114-year-old woman. In addition, they have succeeded in extending the telomeres in these cells. During our lifetime, the telomeres that protect our DNA become shorter and shorter during cell division, which at some point can damage the genes. This can cause aging diseases. By lengthening the telomeres in the pluripotent cells, the DNA is well protected again. With this, the scientists “rejuvenate” the cell.
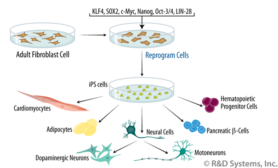
Image 8: Directed Differentiation of iPS Cells. (credits naar Figure adapted from Amabile, G. & A. Meissner (2009) Trends Mol. Med. 15:59.)
Scientists have learned from the suspended animation of tardigrades how to make vaccines more durable, so that they do not always have to be kept refrigerated. If the vaccine is not yet needed, scientists can let the cells in a vaccine dry out. Later, when the vaccine is needed, the cells in the vaccine can be reactivated by hydrating them. This method can also be used in the preservation of DNA, stem cells and platelets.
So we’ve already learned quite a bit from these animals, and according to Rinny there may be more undiscovered immortal animals. Who knows what we can learn from that! Until now, however, most techniques are still in the experimental phase, and therefore mainly applied to mice. If we succeed in creating pluripotent cells in humans, we can prevent or cure many (aging) diseases in combination with the lengthening of the telomeres. In the future, these techniques could increase the average life expectancy of people. We may even achieve immortality one day.
Sources:
- Peniel M. Dimberu, Immortal Jellyfish Provides Clues for Regenerative Medicine, 25 april 2011, https://singularityhub.com/2011/04/25/immortal-jellyfish-provides-clues-for-regenerative-medicine/#:~:text=None%20other%20than%20the%20humble,again%20after%20reaching%20full%20maturity
- Caroline Kraaijvanger, ongelooflijk: zeeslakken onthoofden zichzelf en regenereren vervolgens een compleet nieuw lichaam, 9 maart 2021, https://www.scientias.nl/ongelofelijk-zeeslakken-onthoofden-zichzelf-en-regenereren-vervolgens-een-compleet-nieuw-lichaam/
- Vivian Lammerse, wetenschappers reprogrammeren de cellen van 114-jaar oude vrouw, 25 maart 2020, https://www.scientias.nl/wetenschappers-reprogrammeren-de-cellen-van-114-jaar-oude-vrouw/
- Ronald S. Petralia, Mark P. Mattson, Pamela J.Yao, Aging and longevity in the simplest animals and the quest for immortality, 5 juni 2014, https://www-sciencedirect-com.proxy.library.uu.nl/science/article/pii/S1568163714000622
- Ylva Poelman, Beerdiertjes zijn waarschijnlijk de meest mishandelde organismen op aarde, 5 december 2017, https://www.trouw.nl/nieuws/beerdiertjes-zijn-waarschijnlijk-de-meest-mishandelde-organismen-op-aarde~b88cf954/?referrer=https%3A%2F%2Fwww.google.com%2F
Images:
- Image 1: https://oceanexplorer.noaa.gov/okeanos/explorations/ex1706/dailyupdates/dailyupdates.html#cbpi=july23.html
- Image 2: https://inhabitat.com/11000-year-old-deep-sea-animal-fascinates-scientists/
- Image 3: https://www.sonybiotechnology.com/us/blog/induced-pluripotent-stem-cells-show-potential-for-autologous-stem-cell-therapies/
- Image 4: https://immortal-jellyfish.com/
- Image 5: https://www.quora.com/Is-it-true-that-the-only-immortal-living-thing-on-Earth-is-a-specie-of-jellyfish-called-turritopsis-nutricula
- Image 6: https://www.scientias.nl/ongelofelijk-zeeslakken-onthoofden-zichzelf-en-regenereren-vervolgens-een-compleet-nieuw-lichaam/
- Image 7: https://www.nemokennislink.nl/publicaties/beresterk-bodemdiertje-overleeft-overal/
- Image 8: https://www.rndsystems.com/resources/articles/differentiation-potential-induced-pluripotent-stem-cells

