Oceanic Acidification
There’s something in the water
Over the last 2 years, there have been a lot of climate strikes globally. Maybe you participated in one yourself! Roughly half of the protest signs contain exclamations about CO2, the most important greenhouse gas. The protests are about decreasing the amount of CO2 in the air.
What you learn at school about the greenhouse effect of CO2 is correct: the levels of CO2 rise dramatically fast because of the excessive burning of fossil fuels. This results in temperature risings, which have consequences for life on earth. But this is just part of the story. CO2 is not only in the air; it is mostly present in the oceans (95%!). And, higher levels of CO2 in the atmosphere result in even more CO2 dissolving in the oceans.
This is called ocean acidification, a very unknown subject about which I will tell you the most important things. How does it work? Will you be able to swim in an ocean that is acidic? And is there a solution?


Figure 1: Climate strikes and protest signs.
Firstly, how does CO2 dissolve in water? Most of us are familiar with opening a Coca Cola bottle and seeing the CO2 bubbles being released from the solution. So, it seems weird that CO2 would dissolve in in the ocean, without a cap to ‘hold the CO2’. However, it does! You should compare the Coca Cola bottle with a bottle of still water (water without bubbles). When looking at the labels, you will discover that the bottles contain an equal amount of CO2. But, when you open the bottles, only the Coca Cola one will have bubbles. The CO2 in still water stays dissolved. This is because of the minerals and salts in the still water, which are present in the ocean as well. So, CO2 can dissolve in the oceans because of minerals and salts.
The fact that CO2 from the atmosphere can dissolve in the oceans, is essential for the climate. By taking up the greenhouse gas, the oceans slow down temperature rising in the atmosphere. However, some chemical reactions take place when CO2 is dissolved in the oceans. CO2 reacts with water and different molecules are formed, one of these molecules is called H+.
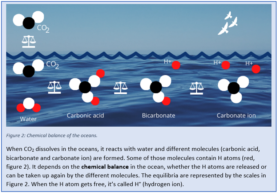
H+ lowers the pH-value of the ocean: it makes the ocean more acidic. The pH-value of the ocean nowadays is 8.1. This means that according to the pH scale, it is not acidic at all. Why then this issue is called ocean acidification? The oceans are basic, but the pH-value becomes increasingly lower, so more acidic than before, which is called acidification.

So, will you dissolve when swimming in an ocean that is more acidic? No! When you look at the pH-value of the ocean and the pH scale, you will understand that the ocean is still basic. However, a lot of animals (for example: crabs and coral reefs) experience problems. They are getting weaker and less abundant. This has consequences for humankind. 1/7th of the world population is dependent on food sources from the ocean. Also, coral reefs play a great role in coastal protection. Do you want to know more about coral reefs? Read Anne’s article on the blog!

But then, is there a solution to ocean acidification? Yes, says Prof. Dr. Gert Jan Reichart. A way to solve this problem is ocean alkalinization. This means that basic substances, such as chalk or crushed rock, are put in the water. The basic molecules will react with H+, so there are less free H+. This will change the pH-value and the chemical balance of the oceans. That way, more CO2 can be taken up from the atmosphere and be dissolved in the water. “The only way to do something about greenhouse gasses and global warming is by changing the chemical balance in the ocean” Moreover, Gert Jan says: “Planting trees has little effect if you look at how much CO2 is taken up by land (almost nothing).” So, this solution would not only counter acidification, but it could also be a solution to global warming. Ocean specialists like Gert Jan are still working on this, but chances are we will see this in the future!
Hopefully now you know more about the oceans and the role they play in the climate. You have learned that ocean acidification is about the oceans not getting acid but becoming less basic. It all has to do with CO2. Increasingly more CO2 is taken up by the oceans because of more CO2 in the atmosphere. This confuses the ocean balance, which is a problem for ocean life and humankind. But the fact that oceans can take up CO2 is essential in slowing down global warming. A solution that goes against acidification and that enlarges CO2 uptake by the oceans, is ocean alkalinization. There is hope… CO2 is in the oceans!
References:
(1) Prof. Dr. Gert Jan Reichart. 2020. Head of the Oceans Systems Department, Royal NIOZ; Professor in Marine Geology, Utrecht University
(2) John P. Rafferty. Ocean acidifcation. March 8, 2020 https://www.britannica.com/science/ocean-acidification (accessed Oct 8, 2020).
(3) Jiang, L.-Q.; Carter, B. R.; Feely, R. A.; Lauvset, S. K.; Olsen, A. Surface Ocean PH and Buffer Capacity: Past, Present and Future. Scientific Reports 2019, 9 (1), 18624. https://doi.org/10.1038/s41598-019-55039-4.

