2024
Keeping nerve cells alive
A recent study by Dong and colleagues shows the working of a mechanism responsible for the dying of nerve cells in patients suffering from frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). These diseases have a lot of victims worldwide and no cure is yet available. Research into finding this cure and keeping the nerve cells alive thus is very important. Nathalie Witjes, who is responsible for the contact with patients and relatives at the Dutch ALS Foundation, gives more context on this matter.
More than 1.5 million people worldwide are victim of FTD and 58000 people suffer from ALS. Symptoms include the progressive decline in memory loss and muscle function, caused by the death of nerve cells. Nathalie Witjes says: “Every time you see these people, you can see the decline [in muscle function].” She elaborates that the loss of muscle function is caused by the fact that the muscles are no longer controlled. This decline can go very fast, but it varies per patient. No cure is available for ALS and FTD, so a lot of research is done in this field. A recent study by Dong and colleagues published by the American Association for the Advancement of Science shows a way that may prevent the death of nerve cells by blocking a mechanism leading up to this.
Nerve cells -or neurons- transport all kinds of information through your body; signals from one part of your brain to the other as well as signals from your brain to your muscles to direct them. It is a very fine network of these nerve cells and if it works well, our body is a well-working machine. However, if part of the network cannot transfer the given signal, for example because the neurons in that part of the “signal highway” are deceased, a lot of different diseases can pop up. These diseases include ALS and FTD.
In development of a disease, most of the time a specific part of someone’s DNA, a gene, is responsible. In specific types of ALS and FTD the same gene is involved. Genes code for proteins, and these proteins fulfill al kinds of tasks in your body concerning function and regulation of tissues and organs, as well as fulfilling an important role in their structure.
The protein which the specific ALS/FTD gene codes for is called poly-GR. This protein is toxic to nerve cells, meaning that eventually, through a series -or pathway- of many other proteins, it can either lead to the less efficient functioning of the cell or even cell death. Such a pathway is like a chain reaction, in which one event causes the next. The chain reaction started by poly-GR will lead to cell death.
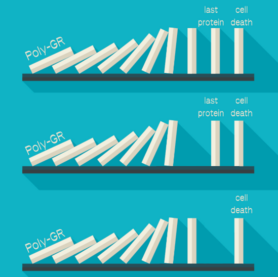 The chain reaction initiated by Poly-GR, which can either lead to cell death or its prevention by the blockage of one of the proteins.(made with Canva)
The chain reaction initiated by Poly-GR, which can either lead to cell death or its prevention by the blockage of one of the proteins.(made with Canva)
The mechanism behind this type of cell death in FTD and ALS was previously unknown, so this discovery by Dong and colleagues opens up a new field of possible treatments. If you want to prevent cell death, some part of the mechanism has to be blocked. All the proteins work in a specific order and if one of them is missing or does not work, the chain reaction cannot continue and the next protein will not be activated. Dong and his colleagues explored different options and one of them is knocking down a protein in the chain reaction, which should normally activate the last protein in the chain. When this last protein is not activated anymore, cell death is prevented from happening. Another option in which cell death is prevented, is by targeting the last protein itself by inhibiting it -or switching it off- with a small particle that could be used as a drug. These possible solutions do not decrease the amount of Poly-GR, but do lower it’s toxic effects.
As with all drug development processes, it is a tedious process. This includes the discovery itself, preclinical research, clinical research and research by the Food and Drug Administration (FDA). Having discovered the mechanism of cell death in certain types of ALS and FTD is an important step in the right direction to making these diseases curable. “Every small discovery gives a spark of hope.” Nathalie Witjes says about the discoveries being done in the field of ALS. Research like that of Dong and colleagues may improve the quality and even duration of the life of patients.
Sources
Accelerating Discovery for Frontotemporal Dementia | Milken Institute. (2024, 15 oktober). Milken Institute. https://milkeninstitute.org/philanthropy/science-philanthropy-accelerator-research-and-collaboration-sparc/neuroscience/frontotemporal-dementia#:~:text=Overview,to%20be%20living%20with%20FTD
Amyotrophic lateral sclerosis (ALS). (z.d.). National Institute Of Neurological Disorders And Stroke. https://www.ninds.nih.gov/health-information/disorders/amyotrophic-lateral-sclerosis-als#:~:text=ALS%20is%20progressive%2C%20meaning%20the,reverses%20the%20progression%20of%20ALS.
The Drug Development Process. (2018, 4 October). U.S. Food & Drug Administration. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process
What Are Frontotemporal Disorders? Causes, Symptoms, and Treatment. (2021, 30 July). National Institute On Aging. https://www.nia.nih.gov/health/frontotemporal-disorders/what-are-frontotemporal-disorders-causes-symptoms-and-treatment#:~:text=So%20far%2C%20there%20is%20no,disorders%20can%20help%20guide%20treatment.
What are proteins and what do they do?: MedlinePlus Genetics. (z.d.). https://medlineplus.gov/genetics/understanding/howgeneswork/protein/
Dong, D., Zhang, Z., Li, Y., Latallo, M. J., Wang, S., Nelson, B., Wu, R., Krishnan, G., Gao, F., Wu, B., & Sun, S. (2024). Poly-GR repeats associated with ALS/FTD gene C9ORF72 impair translation elongation and induce a ribotoxic stress response in neurons. Science Signaling, 17(848). https://doi.org/10.1126/scisignal.adl1030
Newman, T. (2023, 18 juli). All you need to know about neurons. https://www.medicalnewstoday.com/articles/320289#neurons_look_like
Wolfson, C., Gauvin, D. E., Ishola, F., & Oskoui, M. (2023). Global Prevalence and Incidence of Amyotrophic Lateral Sclerosis. Neurology, 101(6). https://doi.org/10.1212/wnl.0000000000207474
You must be logged in to post a comment.

